Physics
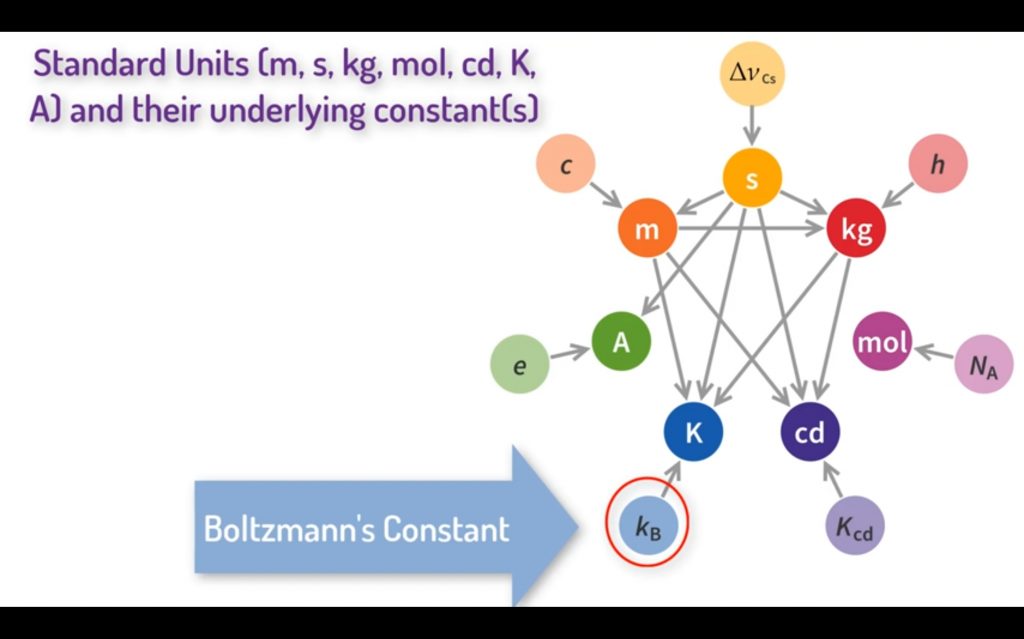
Standard Units
Ultraviolet catastrophe
The ultraviolet catastrophe, also called the Rayleigh–Jeans catastrophe, was the prediction of late 19th century/early 20th century classical physics that an ideal black body at thermal equilibrium would emit an unbounded quantity of energy as wavelength decreased into the ultraviolet range.
The term “ultraviolet catastrophe” was first used in 1911 by Paul Ehrenfest, but the concept originated with the 1900 statistical derivation of the Rayleigh–Jeans law. The phrase refers to the fact that the Rayleigh–Jeans law accurately predicts experimental results at radiative frequencies below 100 THz, but begins to diverge from empirical observations as these frequencies reach the ultraviolet region of the electromagnetic spectrum.
Since the first use of this term, it has also been used for other predictions of a similar nature, as in quantum electrodynamics and such cases as ultraviolet divergence.
Black body

A black body radiator used in CARLO laboratory in Poland. It is an approximation of a model described by Planck’s law utilized as a spectral irradiance standard.

As the temperature of a black body decreases, its intensity also decreases and its peak moves to longer wavelengths. Shown for comparison is the classical Rayleigh–Jeans law and its ultraviolet catastrophe.
A black body or blackbody is an idealized physical body that absorbs all incident electromagnetic radiation, regardless of frequency or angle of incidence. The name “black body” is given because it absorbs all colors of light. A black body also emits black-body radiation. In contrast, a white body is one with a “rough surface that reflects all incident rays completely and uniformly in all directions.”
A black body in thermal equilibrium (that is, at a constant temperature) emits electromagnetic black-body radiation. The radiation is emitted according to Planck’s law, meaning that it has a spectrum that is determined by the temperature alone (see figure at right), not by the body’s shape or composition.
An ideal black body in thermal equilibrium has two main properties:
- It is an ideal emitter: at every frequency, it emits as much or more thermal radiative energy as any other body at the same temperature.
- It is a diffuse emitter: measured per unit area perpendicular to the direction, the energy is radiated isotropically, independent of direction.
An approximate realization of a black surface is a hole in the wall of a large insulated enclosure (an oven, for example). Any light entering the hole is reflected or absorbed at the internal surfaces of the body and is unlikely to re-emerge, making the hole a nearly perfect absorber. When the radiation confined in such an enclosure is in thermal equilibrium, the radiation emitted from the hole will be as great as from any body at that equilibrium temperature.[further explanation needed]
Real materials emit energy at a fraction—called the emissivity—of black-body energy levels. By definition, a black body in thermal equilibrium has an emissivity ε = 1. A source with a lower emissivity, independent of frequency, is often referred to as a gray body. Constructing black bodies with an emissivity as close to 1 as possible remains a topic of current interest.
In astronomy, the radiation from stars and planets is sometimes characterized in terms of an effective temperature, the temperature of a black body that would emit the same total flux of electromagnetic energy.
Thermal equilibrium
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be in thermal equilibrium with itself if the temperature within the system is spatially uniform and temporally constant.
Systems in thermodynamic equilibrium are always in thermal equilibrium, but the converse is not always true. If the connection between the systems allows transfer of energy as ‘change in internal energy‘ but does not allow transfer of matter or transfer of energy as work, the two systems may reach thermal equilibrium without reaching thermodynamic equilibrium.
Maxwell–Boltzmann distribution
https://en.wikipedia.org/wiki/Maxwell%E2%80%93Boltzmann_distribution
Copenhagen (play)
Unitary matrix
Four-vector
Free particle
In physics, a free particle is a particle that, in some sense, is not bound by an external force, or equivalently not in a region where its potential energy varies. In classical physics, this means the particle is present in a “field-free” space. In quantum mechanics, it means the particle is in a region of uniform potential, usually set to zero in the region of interest since the potential can be arbitrarily set to zero at any point in space.